Aquarium Water Chemistry
To maintain a successful aquarium, you must have a fundamental understanding of the chemistry of your fishes environment, that is water.
This section introduces to water hardness, acidity, alkalinity, salinity and nitrites and nitrates.
Rest assured this will not be hard, but it is something that you need to know and how to measure.
The Nitrogen Cycle
Introduction
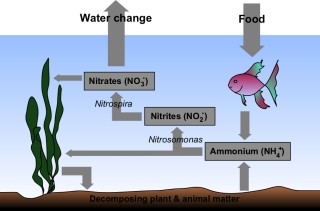
There is a bit of chemistry happening in your aquarium which is performed between you, your fish and bacteria.This is known by many different names such as: nitrogen cycle, cycling, nitrification and biological cycle.
This is a finally balanced system and not understanding the nitrogen cycle is the commonest reason for loss of fish.
The Stages
- Excretion and uneaten food in the tank creates ammonia. This is highly toxic and will soon kill your fish
- Bacteria in the water (Nitrosomonas) oxidize the ammonia and create nitrite. This is also very dangerous for fish
- Another bacteria (Nitrobacter) converts the nitrites into nitrates, which are a lot less harmful
- You performing water changes reduce the amount of nitrites in the water making it safe for the fish
Establishing The Cycle
When a tank is new this cycle has not yet been created, there are solutions available to help establish this quicker, but the best way is to be patient and do not add all the fish at once. If you do the system will not be able to cope with the ammonia produced and the fish will poison themselves.Slowly adding fish allows both Nitrosomonas and Nitrobacter to multiply to the correct levels to dispose of the ammonia.
Water Changes
Getting in the routine of regular water changes removes the nitrates which, although less harmful than ammonia and nitrite can still prove lethal in large doses.Acidity And Alkalinity

Introduction
In the natural environment, the one factor that normally has the most effect on local water conditions is the substrate and rock that reside in it.As an example water that runs through limestone will be alkaline and water that is from a peaty environment with be acidic.
To ensure that your fish are healthy you must measure the water's acidity or alkalinity levels and make sure they are within tolerable limits.
Measurement
The measurement of acidity or alkalinity is made in units called pH (pondus hyrogennii) you don't need to know this, but it is the measure of the weight of hydrogen ions in the water sample.)- A pH reading of 7 means the water is neutral
- A pH reading of less than 7 means the water is acidic
- A pH reading of above 7 means the water is alkaline
The one thing that you should be aware of is that this is not a sliding scale, each incremental step indicates an increment of ten times the previous. So what appear small insignificant differences aren't!!
To help you monitor the water pH testing kits are readily available, ranging from simple litmus trips to electronic readers.
Hardness

Introduction
Like acidity and alkalinity water hardness is determined by the substrate. Hardness is created by metallic ions primarily calcium.The subject requires a much fuller explanation than the brief summary below, but this should get you started!
One easy point to remember is that the hardness of marine water is always high.
Measurement
There are various measurements for water hardness below are those that you will most likely see.
- kH - is the measurement of the concentration of bicarbonate and carbonate ions
- gH - is the measurement of magnesium and calcium ions in the water (General hardness)
- dH - degrees of hardness
- PPM - parts per million
There are testing kits widely available and the table below will hopefully help you make sense of their readings.
| DH | PPM | Water Hardness |
| 0-3 | 0-54 | soft |
| 3-6 | 54-108 | slightly soft |
| 6-12 | 108-216 | slightly hard |
| 12-18 | 216-324 | moderately hard |
| 18-30 | 324-540 | hard |
Salinity

Introduction
Salinity is something that you only really need to worry about if you are keeping a marine or brackish water system.Compared to the other sections this is all pretty straightforward.
Simply put salinity is the measurement of the amount of salt present in water.
Measurement
To calculate the amount of salt there is in a water sample you must test its specific gravity (SG).Specific gravity is the ratio of the density of a sample compared against distilled water at 4C (39F) (which has a reading of 1.000).
Marine water should typically be between the ranges of 1.020 - 1,027. Brackish water will lie in between 1.000 and 1.020.

Hydrometer
To measure the specific gravity you need a hydrometer.The simplest form is a tube that floats in the water, and as salt water is easier to float in it will rise slightly higher than in freshwater.